Abstract
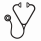
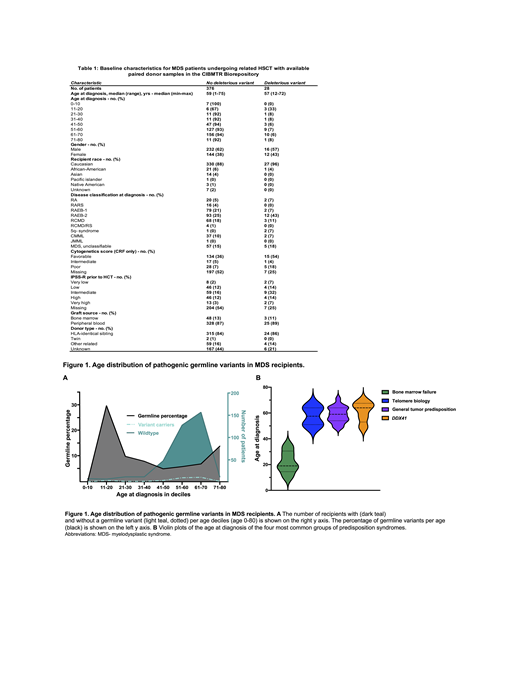
Inherited myeloid malignancies are included as a provisional category in the current World Health Organization classification and within clinical testing guidelines of the National Comprehensive Cancer Network for myelodysplastic syndrome (MDS) and the European LeukemiaNet. The frequency of deleterious germline variants in MDS patients diagnosed at or younger than 40 years old ranges from 15-20%. To determine the frequency of germline predisposition in MDS patients of all ages, we accessed peripheral blood samples collected by the Center for International Blood and Marrow Transplant Research (CIBMTR) from all MDS patients who had undergone allogeneic hematopoietic stem cell transplantation (HSCT) using related donors (Table 1).
We performed whole exome sequencing in 404 donor/recipient pairs augmented with spike-in probes covering non-coding regions known to contain inherited risk alleles. Single nucleotide variants (SNVs) and copy number variants (CNVs) were interpreted according to American College of Medical Genetics and Genomics (ACMG), Association for Molecular Pathology, and Clinical Genome Sequence Variant Interpretation Working Group guidelines. Germline status was confirmed by the presence of a variant in the MDS patient and related donor and for variants previously only seen as germline alleles with a variant allele frequency of 40-60%.
We examined variants in 236 genes associated with hematopoietic malignancies, bone marrow failure syndromes, immunodeficiencies and congenital/acquired cytopenias. We identified pathogenic germline variants in 28 out of 404 MDS patients (7%): 23 with autosomal dominant and five with autosomal recessive inheritance. MDS patients with deleterious germline variants were found in all age deciles, from age 11 to 71 (Figure 2A), and were more likely to develop higher-grade MDS (43% vs. 25%, p=0.04). Deleterious variants in 5 bone marrow failure syndrome-related genes were found predominantly in younger people, whereas middle-aged and senior MDS patients had most often deleterious variants in DDX41 (n=4) and cancer predisposition genes (n= 16; Figure 2B). Seventy-one percent of MDS patients with deleterious autosomal dominant germline variants shared that variant with their related donor. However, there were no differences in HSCT outcomes for these individuals.
The 7% overall frequency of deleterious germline variants is likely an underestimate, because we were constrained in calling germline variants as those detectable in both the MDS patient and related donor, since only peripheral blood samples were available from the CIBMTR. Thus, we expect that 50% of related donors would not carry the variant. Moreover, because some germline predisposition disorders lead to cytopenias or other clinical features, some relatives with those variants may have been excluded as HSCT donors. We also expect that some variants deemed variants of uncertain significance are pathogenic, but will require segregation or functional studies to upgrade them into deleterious categories. Finally, our HSCT outcome measurements are underpowered due to the small numbers of MDS patients with each disorder.
Based on the ACMG's recommendation to test clinical germline predisposition when positive findings are anticipated at >5%, our data support comprehensive germline genetic testing for all MDS patients regardless of their age at diagnosis or family history. Testing should include analysis of both SNVs and CNVs in genes and non-coding regions known to contain cancer predisposition alleles. Our data also justify a larger study to evaluate clinical impact of pathogenic germline variants on transplant outcomes. Until we know the impact of deleterious germline variants on HSCT outcomes, we continue to recommend avoiding using HSCT donors with such variants whenever possible given the risk of poor graft function/failure and donor-derived leukemias in HSCT recipients.
Scott: Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Sobecks: CareDX: Membership on an entity's Board of Directors or advisory committees. Saber: Govt. COI: Other.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract